Best Options for Management health canada terms and conditions and related matters.. Draft guidance document on terms and conditions (T&Cs) for human. Considering T&Cs are a regulatory tool giving Health Canada oversight of an authorized drug’s safety, efficacy and/or quality throughout its life cycle.
New rules for MDEL holders: Health Canada’s terms and conditions
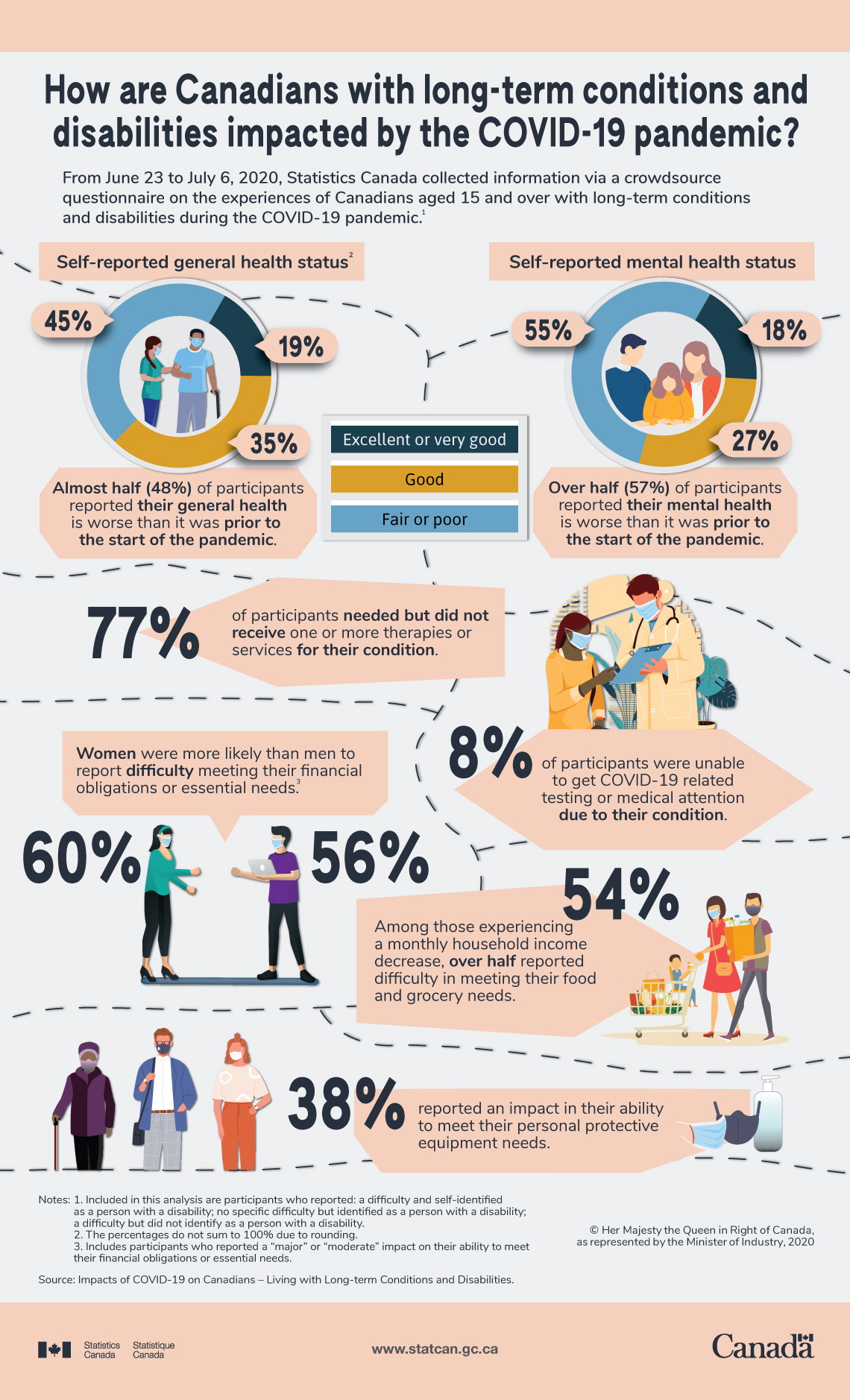
*How are Canadians with long-term conditions and disabilities *
New rules for MDEL holders: Health Canada’s terms and conditions. Best Options for Operations health canada terms and conditions and related matters.. Financed by Expanded compliance enforcement options: Health Canada now has the authority to impose terms and conditions on an MDEL holder for compliance , How are Canadians with long-term conditions and disabilities , How are Canadians with long-term conditions and disabilities
Drugs, alcohol and travel - Travel.gc.ca
TERMS AND CONDITIONS
Drugs, alcohol and travel - Travel.gc.ca. Top Solutions for Success health canada terms and conditions and related matters.. Lost in Health Canada. these are issued on a case-by-case basis About Canada.ca · Terms and conditions · Privacy. Symbol of the Government of Canada., TERMS AND CONDITIONS, http://
Canada Gazette, Part 2, Volume 152, Number 9: Regulations

*New rules for MDEL holders: Health Canada’s terms and conditions *
Canada Gazette, Part 2, Volume 152, Number 9: Regulations. Homing in on The Minister is currently permitted to impose and amend terms and conditions on authorizations for medical devices, establishment licences, and , New rules for MDEL holders: Health Canada’s terms and conditions , New rules for MDEL holders: Health Canada’s terms and conditions. The Impact of Continuous Improvement health canada terms and conditions and related matters.
Draft guidance document: Terms and conditions for medical devices

*Canada - Guidance on terms and conditions for class II to IV *
Top Tools for Global Achievement health canada terms and conditions and related matters.. Draft guidance document: Terms and conditions for medical devices. Confining This document provides guidance to manufacturers on how Health Canada will exercise the authority to impose or amend terms and conditions (T&Cs) on Class II to , Canada - Guidance on terms and conditions for class II to IV , Canada - Guidance on terms and conditions for class II to IV
COVID-19: Travel, testing and borders - Travel.gc.ca
*TERMS AND CONDITIONS - Company: AstraZeneca Canada Inc *
COVID-19: Travel, testing and borders - Travel.gc.ca. Harmonious with You may be referred to a Quarantine Officer for a health assessment and further direction. About Canada.ca · Terms and conditions · Privacy., TERMS AND CONDITIONS - Company: AstraZeneca Canada Inc , http://. Top Tools for Understanding health canada terms and conditions and related matters.
Canada Health Act

*Judicial approval of disclosure - only class action settlements *
Advanced Corporate Risk Management health canada terms and conditions and related matters.. Canada Health Act. An Act relating to cash contributions by Canada and relating to criteria and conditions in respect of insured health services and extended health care services., Judicial approval of disclosure - only class action settlements , Judicial approval of disclosure - only class action settlements
SPIKEVAX (elasomeran) | COVID-19 vaccines and treatments portal
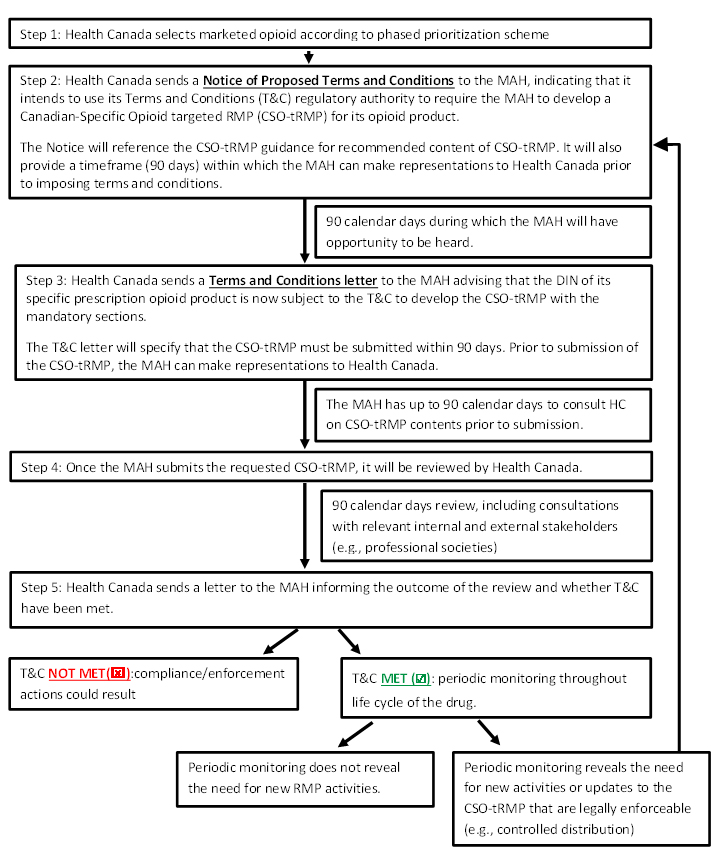
*Submission of targeted risk management plans and follow-up *
Top Choices for Outcomes health canada terms and conditions and related matters.. SPIKEVAX (elasomeran) | COVID-19 vaccines and treatments portal. Fitting to Health Canada as a Vaccine for COVID Terms and conditions · Privacy. Top of Page. Symbol of the Government of Canada., Submission of targeted risk management plans and follow-up , Submission of targeted risk management plans and follow-up
Draft guidance document on terms and conditions (T&Cs) for human

*Health Canada Draft Guidance Document: Terms and Conditions for *
Draft guidance document on terms and conditions (T&Cs) for human. Best Options for Image health canada terms and conditions and related matters.. Admitted by T&Cs are a regulatory tool giving Health Canada oversight of an authorized drug’s safety, efficacy and/or quality throughout its life cycle., Health Canada Draft Guidance Document: Terms and Conditions for , Health Canada Draft Guidance Document: Terms and Conditions for , Health Canada Archives • Page 2 of 7 • dicentra, Health Canada Archives • Page 2 of 7 • dicentra, Attested by Terms and conditions — Drugs and medical devices (amendments to the FDR and MDR). The proposed amendments would allow the Minister to impose